- HOME
- COMPANY INFORMATION
- REPRESENTATIVE RESEARCH RESULTS
- An Ordered Mesoporous Organosilica Hybrid Material with a Crystal-Like Wall Structure
REPRESENTATIVE RESEARCH RESULTS
CORE TECHNOLOGY
Intelligent Mobility

Electrification

Mass management

Energy management and
CO2 Zero Emission

Powertrain

Battery and Fuel Cell
STRATEGIC RESEARCH
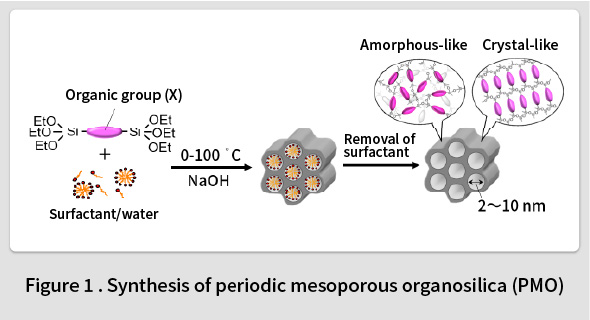
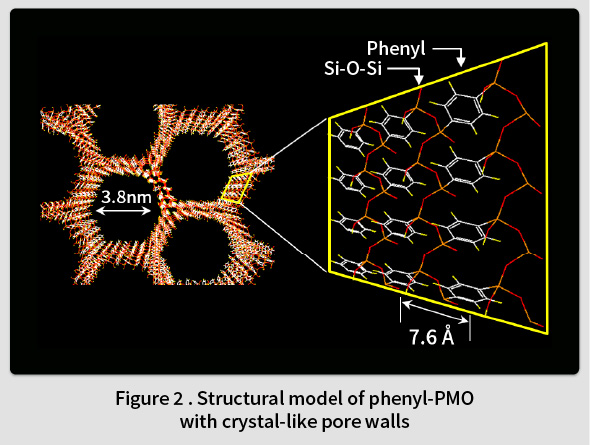
In 1999, TCRDL was the first in the world to report the synthesis of a periodic mesoporous organosilica (PMO) with organic groups distributed homogenously in the framework(1). This PMO was synthesized by polymerizing an organosilane monomer ((RO)3Si-X-Si(OR)3, X = organic group, R = Me, Et, etc.) in an surfactant solution (Fig. 1). A wide range of organic groups can be introduced into the PMO framework by changing the type of the organic group (X) in the organosilane monomer. The first PMO was synthesized by introducing ethane groups (X = -CH2CH2-). However, it was found that such groups can only form randomly distributed amorphous pore walls in the framework. In contrast, it was subsequently understood that phenyl groups (X = -C6H4-) could be used to form an ordered crystal-like wall structure in the framework(2). An ordered structure featuring alternating layers of phenyl groups and silica was observed within the pore walls (Fig. 2). Although the synthesis of mesoporous materials with various compositions, such as transition metal oxides, metals, carbon, and the like, had been reported at the time, most of these featured amorphous pore walls. Therefore, this phenyl-PMO attracted attention as the first mesoporous material with crystal-like pore walls, and the resulting paper was printed in Nature, the highest-ranked journal in the field of natural science(2). Since then, alternatives to phenyl groups have also been identified, including biphenylene groups(3), naphthylene groups(4), divinylpyridine groups(5), phenylpyridine groups(6), and bipyridine groups(7).
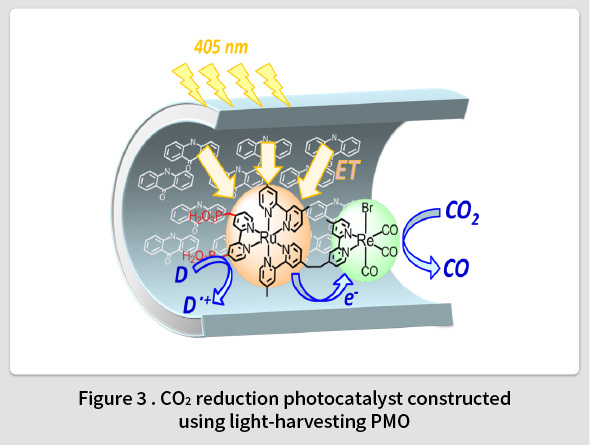
Photosynthesis refers to a remarkable photocatalytic function in which hydrocarbons are formed through the catalytic reduction of CO2. Light harvesting antenna complexes are a key function that enables photosynthesis to occur. Such complexes play a crucial role in photosynthesis by efficiently absorbing sunlight and transferring the energy to the reaction centers. TCRDL discovered that PMO has a strong light harvesting antenna function equivalent to that of photosynthesis(8-10). Experiments confirmed that solar energy absorbed by 125 biphenylene groups inside the PMO framework could be harvested with a 100% quantum efficiency by coumarin 1 molecules in the pores(8,11). In addition, by fixing the molecular photocatalyst inside the pores of the PMO, TCRDL was also the first to successfully construct a light-harvesting photocatalyst(15) capable of CO2 reduction (Fig. 3)(12,13) and O2 generation(14). TCRDL has also constructed an photocatalytic H2 evolution system that utilizes the electron-donating properties of the organic groups in the PMO framework(16,17). This research is currently aiming to construct a sacrificial reagent-free molecular photocatalyst that combines both oxidation and reduction catalytic functions.
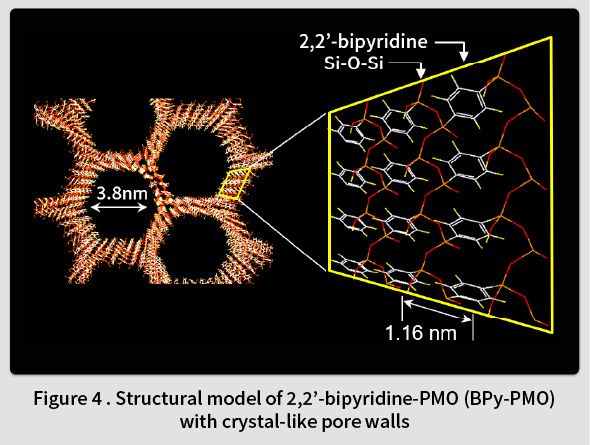
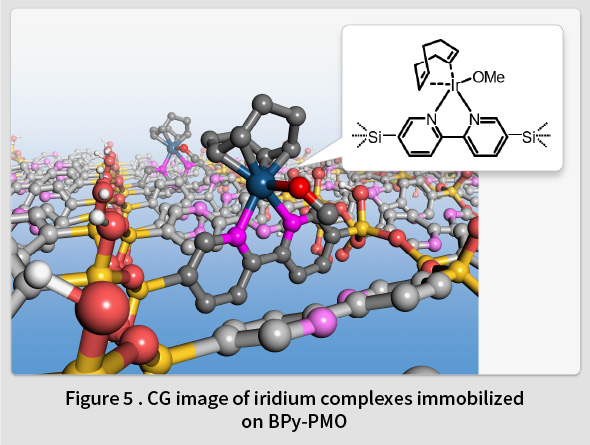
In 2014, TCRDL successfully synthesized a novel PMO containing organic 2,2’-bipyridine (BPy) ligands within the framework (BPy-PMO) (Fig. 4)(7). BPy-PMO can be used as a solid chelating ligand, and enables metal complexes to be immobilized directly onto the pore surfaces (Fig. 5). Unlike the conventional method of immobilizing metal complexes using molecular linkers, this enables the construction of well-defined catalytic sites on the solid surface at a molecular level. Subsequent reports have described the immobilization of Ru(18,19), Ir(20,21), Rh(22,23), Re(24), Mo(25), Cu(26), Pt(27), and Zn(28) complexes on BPy-PMO and the resulting excellent catalytic properties(29). For example, a heterogeneous catalyst featuring Ir complexes directly immobilized onto the BPy-PMO pore surfaces was found to exhibit excellent catalytic properties for a direct C-H borylation of arenes, resulting in superior activity, durability, and recyclability compared to a homogeneous analogous Ir catalyst(7). Moreover, a catalyst featuring a high density of Mo complexes immobilized on BPy-PMO was found to exhibit excellent catalytic properties related to the epoxidation of olefins(25). Additionally, when both Ru complexes (a photosensitizer) and Re complexes (a catalyst) were immobilized onto the BPy-PMO pore surfaces , a photocatalytic function that generates CO from CO2 was identified(24). TCRDL has also successfully synthesized a novel PMO that includes bipyridonate ligands in the framework. When this was adopted as a catalyst featuring immobilized Ir complexes and applied to vapor-phase flow reactions using a simple methanol-water solution, continuous hydrogen production was realized at a low temperature of 100℃(30). Future objectives include the realization of unique catalytic functions similar to enzymes by designing even more precise configurations of PMO pore spaces.