Materials Science and Engineering
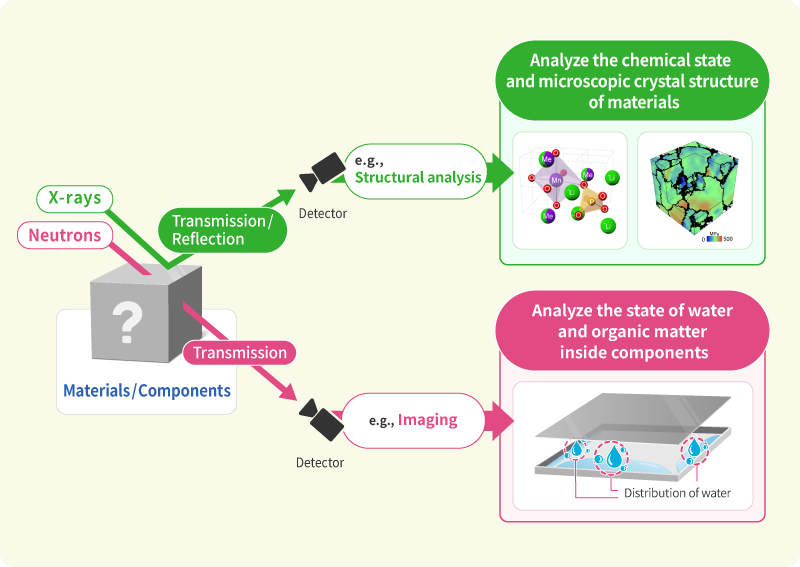
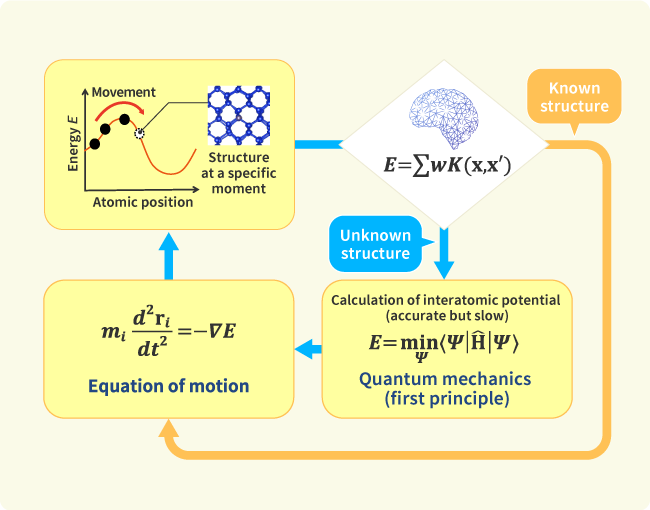
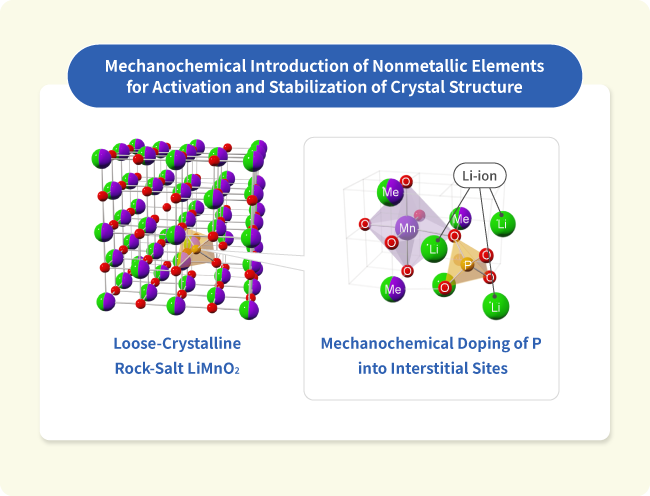
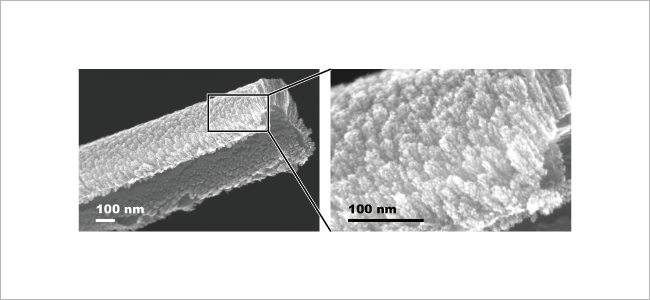
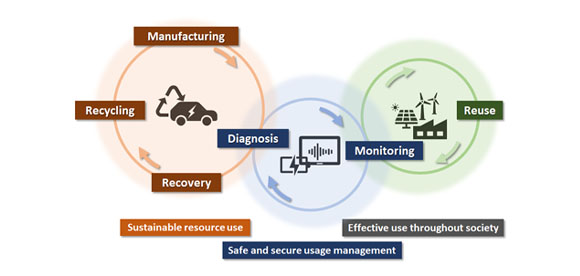
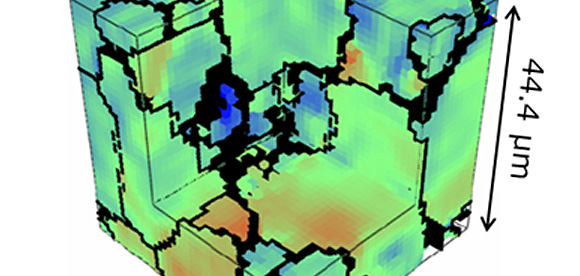
We engage in research and development into the materials that will create breakthroughs in response to increasingly complex social demands, such as the environmental performance and economic rationality required by next-generation automobiles and energy systems. As part of our efforts to create energy storage and conversion materials, in addition to nano-interface reaction design and reaction control technologies, we also advance research and development into technologies that analyze and elucidate phenomena related to material structures and reactive processes at multiple scales using X-rays, light, and quantum beams. Moreover, we work with microfabrication technologies, manufacturing technologies including additive fabrication that integrates material processes and molding, material design at the atomic and molecular level based on computational science, and process development that enables the synthesis of these materials. In addition, we are actively engaged in materials informatics, autonomous and automated experiments, and other forms of fusion research with computer science as a means of dramatically improving the efficiency of materials development.