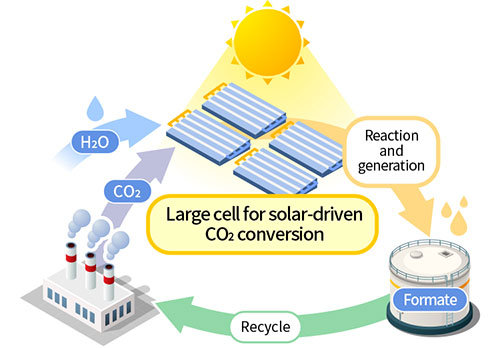
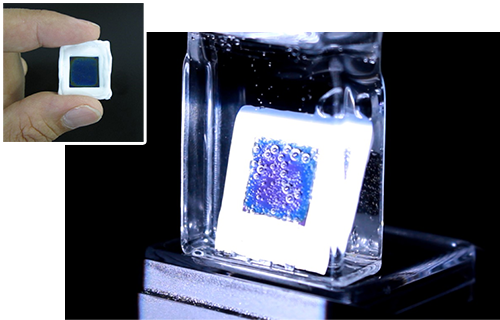
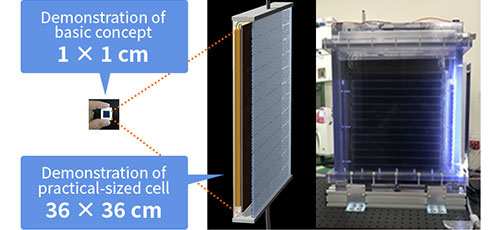
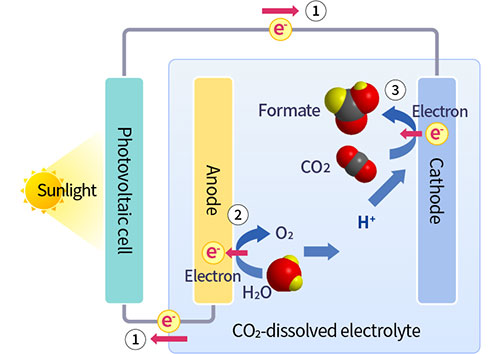
Artificial photosynthesis is a technology that uses solar energy to produce organic compounds from H2O and CO2. In 2011, TCRDL was the first in the world to demonstrate the concept of artificial photosynthetic reactions driven solely by these three elements. This technology is characterized by the use of a semiconductor/metal-complex hybrid catalyst, which has a lower environmental impact because it allows the system to operate at ordinary temperatures and pressures. The solar-to-chemical efficiency* of the technology was 0.04% in 2011. Continuous research and development efforts pushed this efficiency to 4.6% in 2015, which is higher than the solar-to-chemical conversion efficiency of plants.
*This refers to the proportion of energy that can be stored in organic compounds, out of the total solar energy radiated by the sun.
Senior Fellow Takeshi Morikawa: /en/sflabmorikawa/